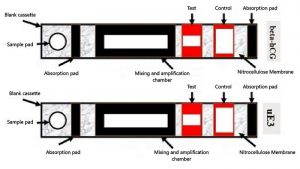
We are presenting our innovative Down syndrome detection kit, specifically developed for early identification of Down syndrome during the first trimester. This kit employs a dual testing approach using biomarkers, specifically free beta-hCG and unconjugated estriol, to improve the accuracy and precision of detecting trisomy 21, surpassing the effectiveness of conventional approaches. This innovative solution eliminates the need for invasive procedures by analyzing maternal urine samples, ensuring safety and comfort for both mother and fetus. Employing colourimetry and fluorimetry principles, our kit delivers rapid and accurate results, empowering expectant parents with vital information about their baby’s health. This device presents significant commercial potential with its non-invasive design, user-friendly interface, and enhanced precision. It offers healthcare professionals and parents a reliable early Down syndrome detection tool, potentially improving prenatal care and informed decision-making. Moreover, its dual detection method and incorporation of fluorescence technology make it a promising option for adoption in clinics, hospitals, and prenatal care facilities globally.